Insilico Medicine Advances AI-Designed IBD Drug ISM5411 to ‘First-in-Human’ Trial

In Brief
ISM5411 is Insilico Medicine’s fifth AI-driven drug program to enter clinical trials, and facilitates the treatment of IBD.

Generative AI-driven clinical-stage biotechnology company Insilico Medicine (“Insilico”) today announced the commencement of the first-in-human study for ISM5411.
Claimed to be a potentially groundbreaking PHD inhibitor for the treatment of inflammatory bowel disease (IBD), ISM5411 is Insilico’s fifth AI-driven drug program to enter clinical trials, marking a significant stride in the company’s innovative drug development initiatives.
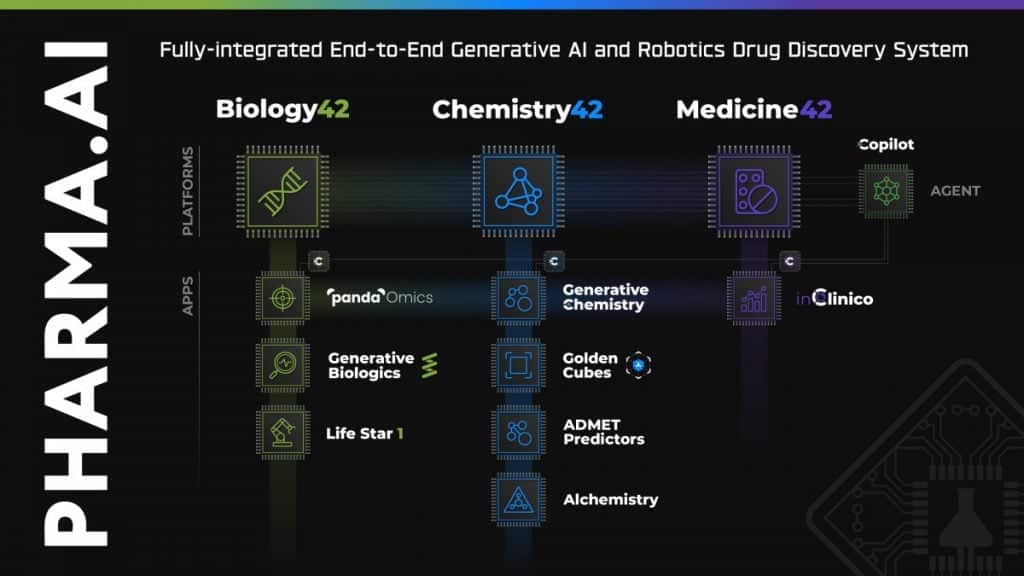
Developed through Insilico’s proprietary end-to-end AI drug discovery platform Pharma.AI, ISM5411showcases the company’s commitment to leveraging cutting-edge technology to address complex medical challenges.
“Insilico’s R&D team used our proprietary generative AI Chemistry42 engine to conduct structure-based small molecule generation based on the PHD target protein structure. After obtaining a series of lead compounds with novel structures, unique binding modes and excellent activity, Insilico’s R&D team carried out further medicinal chemistry optimization and transformation,” Alex Zhavoronkov, PhD, founder and co-CEO of Insilico Medicine told Metaverse Post.
“We ultimately nominated ISM5411, an oral PHD-specific inhibitor with intestinal restriction, as a preclinical candidate,” he added.
Inflammatory bowel disease (IBD) is a chronic inflammatory condition that affects gastrointestinal tract, includes conditions such as ulcerative colitis (UC) and Crohn’s disease (CD). Globally, these diseases impact millions, with 3 million adults affected in the U.S. alone.
With no known cure, IBD patients face an increased risk of colorectal cancer. The current standard of treatment involves anti-inflammatory drugs, providing limited improvement in mucosal healing. Genetic and clinical evidence strongly correlates mucosal healing with positive prognosis.
The ongoing Phase I study aims to assess the safety, tolerability, pharmacokinetics, and food effects of ISM5411, administered at escalating oral doses to 76 healthy subjects. The inaugural dose for healthy volunteers has been successfully administered in Australia, marking a critical milestone in the drug’s development.
To expand the evaluation of ISM5411 to broader populations, Insilico is planning global, multi-center Phase Ib trials.

“In order to evaluate ISM5411 in a wider group, Insilico plans to conduct an international multi-center clinical trial in the US, China, and other locations after the completion of the Phase 1a clinical trial – setting up three treatment groups and one placebo group. The specific dosage will be determined based on the results of the Phase Ia study,” said Insilico’s Zhavoronkov.
Leveraging Generative AI to Accelerate Drug Trials
Insilico’s dedication to addressing unmet clinical needs is evident in the progression of ISM5411, nominated as a preclinical drug candidate in January 2022.
Developed by Chemistry42, Insilico’s generative AI engine for molecule development, ISM5411 boasts a novel scaffold and a unique binding mode. Preclinical studies demonstrated a favorable safety profile and significant anti-colitis efficacy, positioning ISM5411 as an oral, gut-restricted small molecule inhibitor.
Research indicates that Prolyl Hydroxylase (PHD) plays a pivotal role in regulating the stability and transcriptional activity of hypoxia-inducible factor-1α (HIF-1α), which, in turn, drives the expression of barrier-protective genes. PHD is significantly up-regulated in IBD patients, showcasing a strong correlation with inflammatory cytokines and apoptosis markers. This highlights PHD as a potential target for IBD treatment.
Dr. Sujata Rao, MD, Chief Medical Officer at Insilico Medicine, is spearheading the clinical trial and asserts ISM5411 to be impactful for patient population with limited therapeutic options.
“ISM5411 is an oral, PHD-specific drug with intestinal restriction. This offers a key alternative to existing therapies and other therapies in development. An important feature of our AI-designed treatment is its mucosal repair effect. Currently, IBD cannot be cured,” Insilico’s Zhavoronkov told Metaverse Post. “Preclinical data show that ISM5411 simultaneously exhibits anti-inflammatory activity and promotes mucosal repair. It is expected to target the intestinal mucosal repair mechanism and achieve the goal of improving long-term clinical prognosis.”
Insilico Medicine stands as a beacon at the intersection of artificial intelligence and state-of-the-art technologies. With a portfolio exceeding 30 pipelines and four programs in the clinical stage, developed using Pharma.AI, Insilico aims to be a pioneer in AI-driven drug discovery.
The recent launch of the Al R&D Center in Montreal, Canada further fortifies the company’s capabilities in AI algorithm research and platform building.
“Our latest IBD asset and the Company’s fifth in the clinical stage. Our lead drug, for idiopathic pulmonary fibrosis, is the first drug with both an AI-discovered target and designed by AI to reach Phase II trials with patients,” said Insilico’s Zhavoronkov. “And a potentially best-in-class USP1 cancer inhibitor for BRCA-mutated tumors developed by this platform was recently licensed to Exelixis for $80m upfront and additional milestone and royalty payments.”
The Pharma.AI platform was recently updated with new generative AI features — including chat functionality, essentially ChatGPT for drug discovery; virtual kinase panels for off-target screening (called “Golden Cubes”), and an Alchemistry system that calculates free binding energy (BE) for the generated molecules, comparing molecules by BE without any additional physics tools.
Disclaimer
In line with the Trust Project guidelines, please note that the information provided on this page is not intended to be and should not be interpreted as legal, tax, investment, financial, or any other form of advice. It is important to only invest what you can afford to lose and to seek independent financial advice if you have any doubts. For further information, we suggest referring to the terms and conditions as well as the help and support pages provided by the issuer or advertiser. MetaversePost is committed to accurate, unbiased reporting, but market conditions are subject to change without notice.
About The Author
Victor is a Managing Tech Editor/Writer at Metaverse Post and covers artificial intelligence, crypto, data science, metaverse and cybersecurity within the enterprise realm. He boasts half a decade of media and AI experience working at well-known media outlets such as VentureBeat, DatatechVibe and Analytics India Magazine. Being a Media Mentor at prestigious universities including the Oxford and USC and with a Master's degree in data science and analytics, Victor is deeply committed to staying abreast of emerging trends. He offers readers the latest and most insightful narratives from the Tech and Web3 landscape.
More articles

Victor is a Managing Tech Editor/Writer at Metaverse Post and covers artificial intelligence, crypto, data science, metaverse and cybersecurity within the enterprise realm. He boasts half a decade of media and AI experience working at well-known media outlets such as VentureBeat, DatatechVibe and Analytics India Magazine. Being a Media Mentor at prestigious universities including the Oxford and USC and with a Master's degree in data science and analytics, Victor is deeply committed to staying abreast of emerging trends. He offers readers the latest and most insightful narratives from the Tech and Web3 landscape.


















































